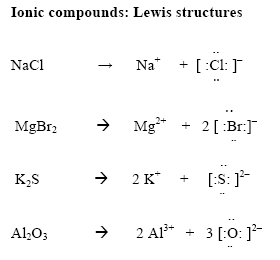Heartwarming Info About How To Draw Ionic Bonds

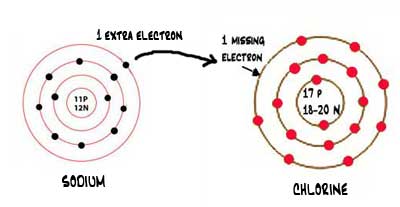
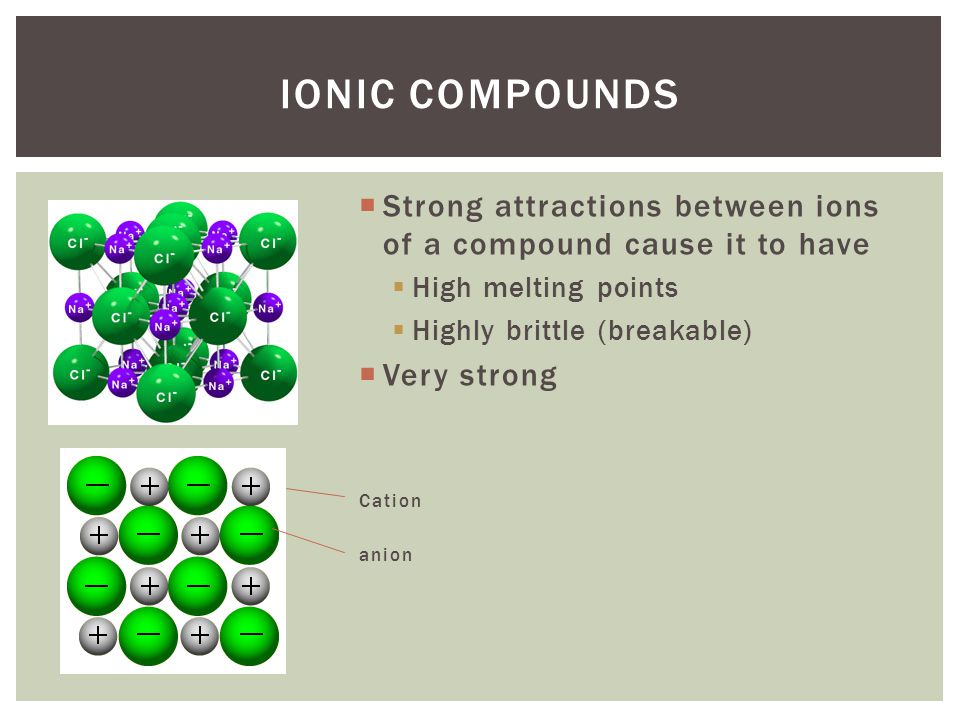
Ionic bonds occur between metals and nonmetals due to the differences in their electronegativity and ionization energy.
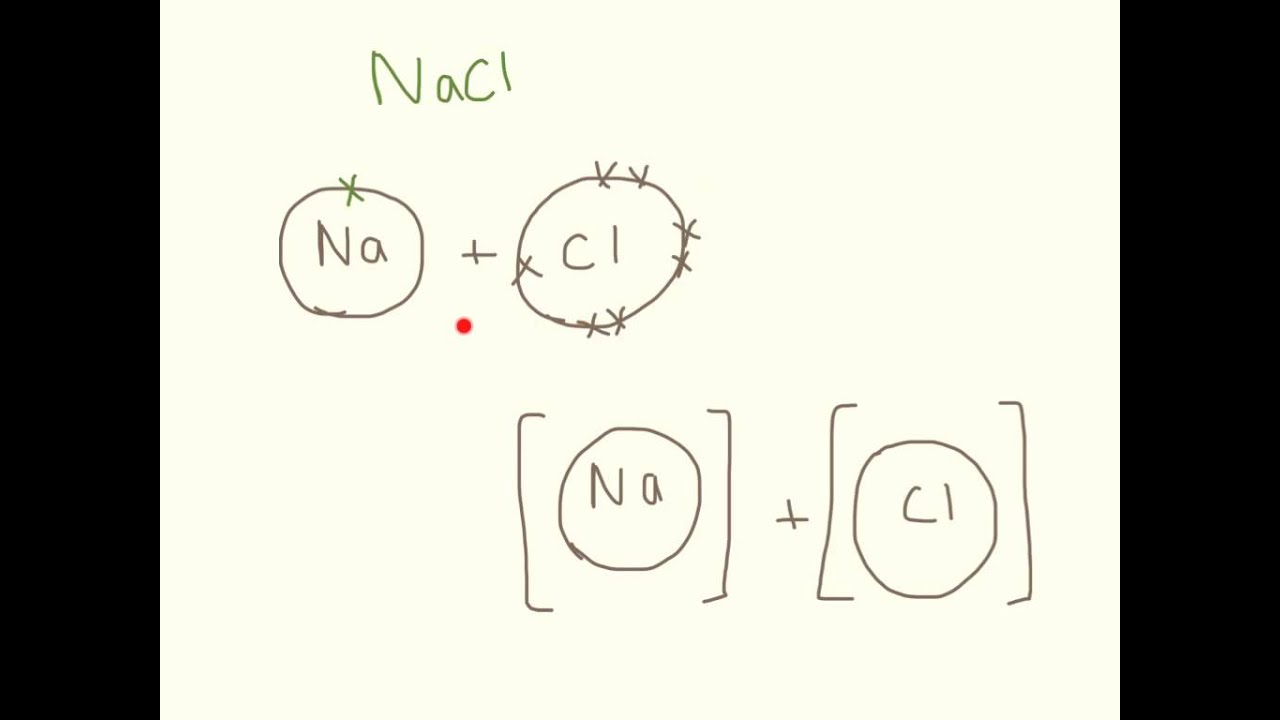
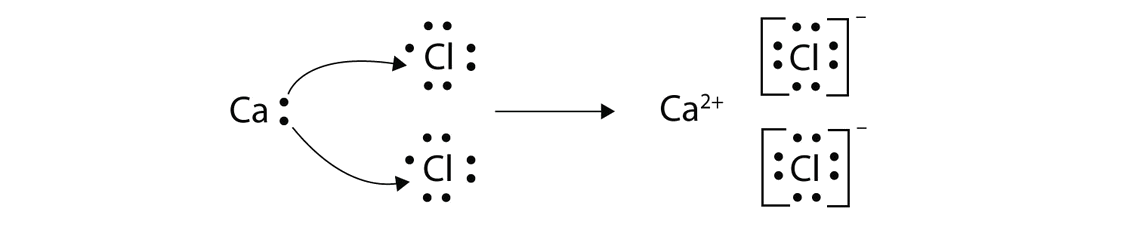
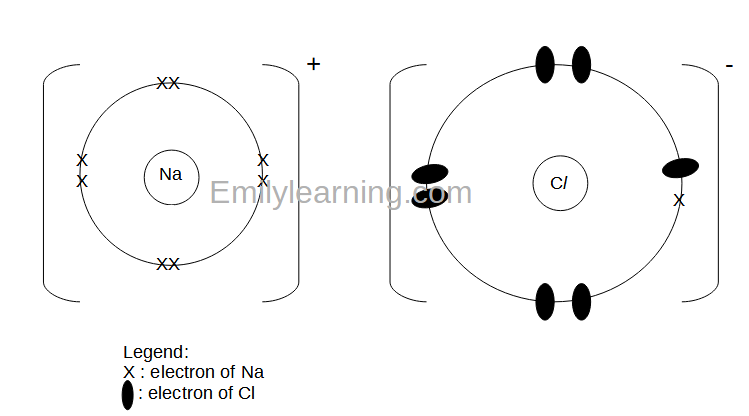
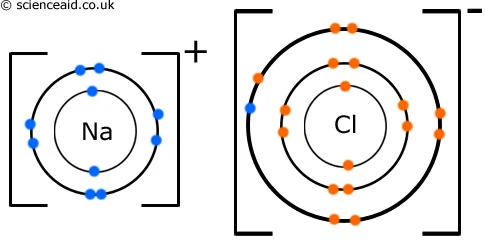
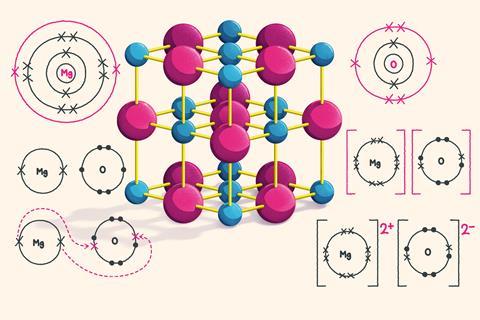
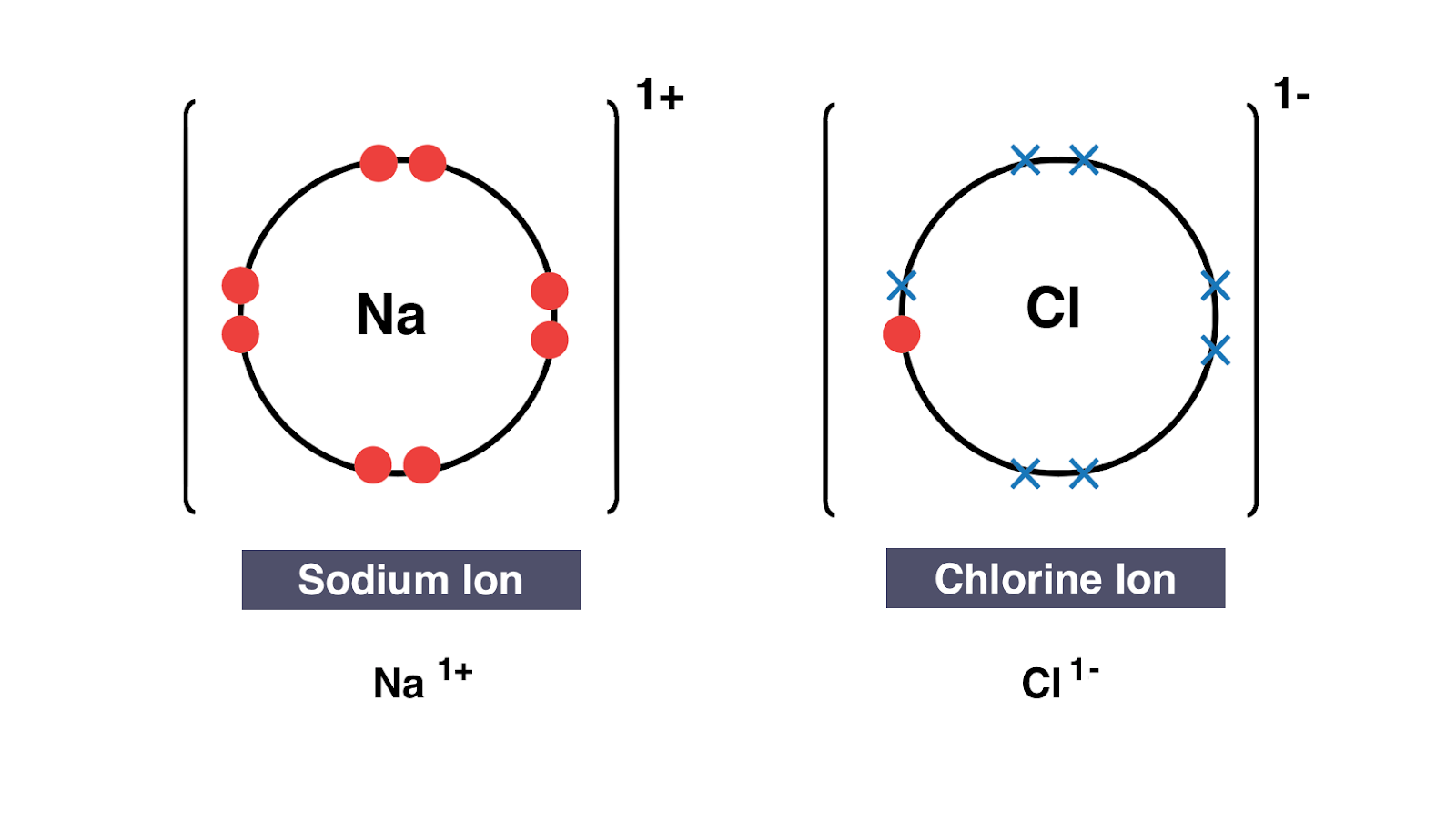
How to draw ionic bonds. The attraction between positive and negatively charged ions creates a chemical bond called an ionic bond. Determine the charge of your ions. First, draw the electron dot diagrams for each.
Anionic bond forms between a metal and a nonmetal. How to draw ionic bondsexample: Draw the lewis structures for each.
This crash course chemistry video tutorial explains the main concepts between ionic bonds found in ionic compounds and polar & nonpolar covalent bonding foun. First, click on the single bond tool to select it. Click on the canvas to drop a single bond.
Even if you don't want to stud. For ionic compounds with polyatomic ions, like nano3 or k2so4, we need to draw the lewis structure for the polyatomic (which is usually a covalent compound where valence. By default, the single bond is formed between two atoms of the first element in.
For each ionic compound, you have been given the element names and the chemical formula. These grades are the stepping stone to your future. An ionic bond occurs when a metal atom loses valence electron (s) to.
A look at ionic bonding, where positive and negative ions attract each other and combine. Drawing ionic bonds (key) introduction: I want to help you achieve the grades you (and i) know you are capable of;