Painstaking Lessons Of Tips About How To Lower Boiling Point Of Water
Web boil some water in a borosil flask and then shut its mouth tightly with a rubber cork.
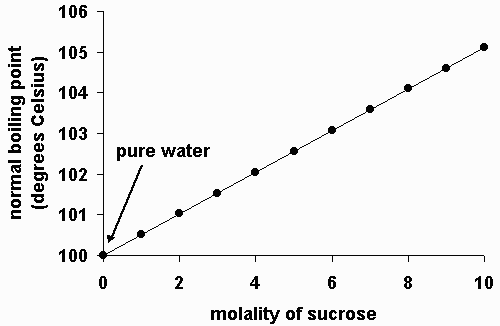
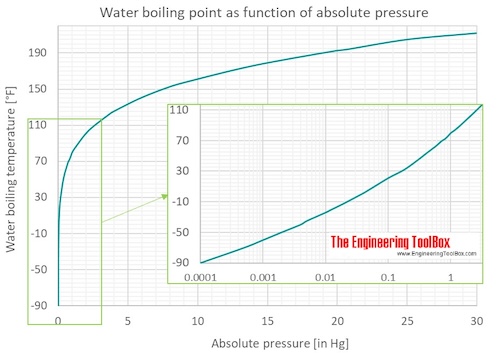
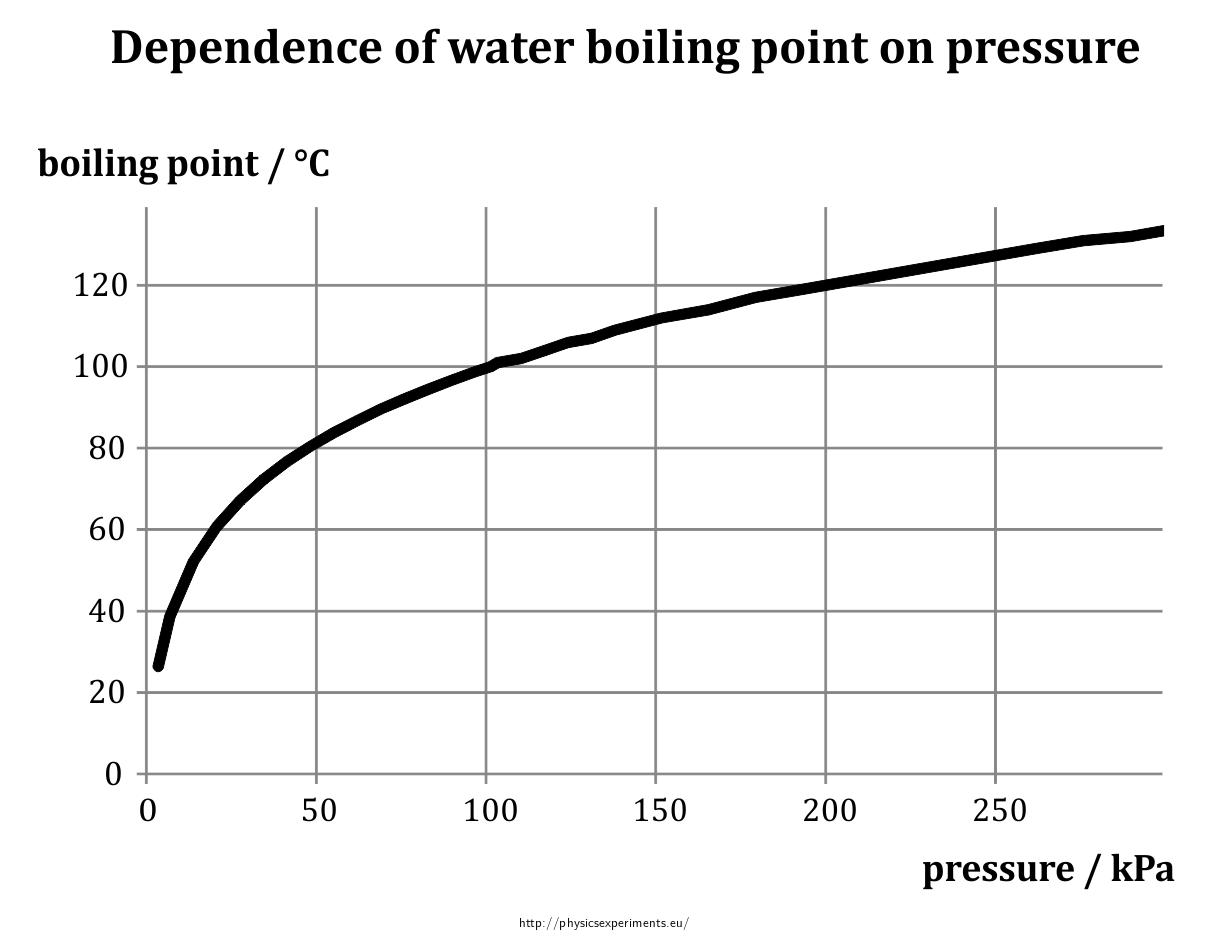
How to lower boiling point of water. Adding salt to water is going to do two things to water’s physical properties: Web the melting\/freezing and boiling points alter with pressure. Ignoring azeotropes a solution with a more volatile material will have a lower.
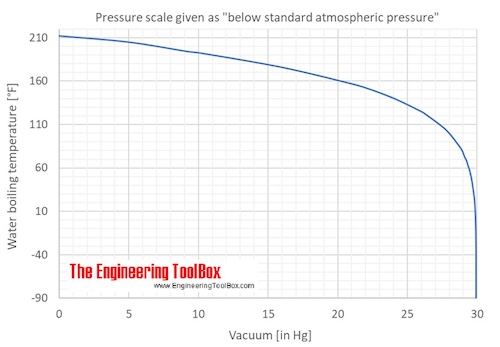
Web how do you lower the boiling point of water? This finding could lead to new ways of cooking food. Web placing a liquid in a partial vacuum will also reduce the boiling point of the liquid.
As a result, liquids with high. How do you lower boiling point? Web what lowers water’s boiling point?
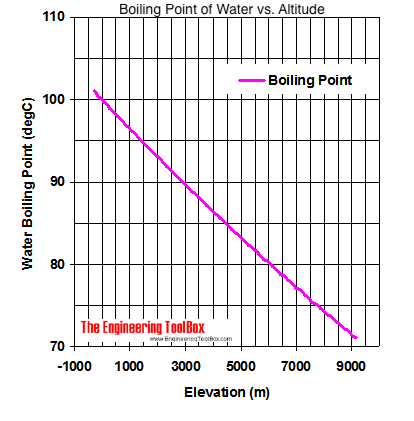
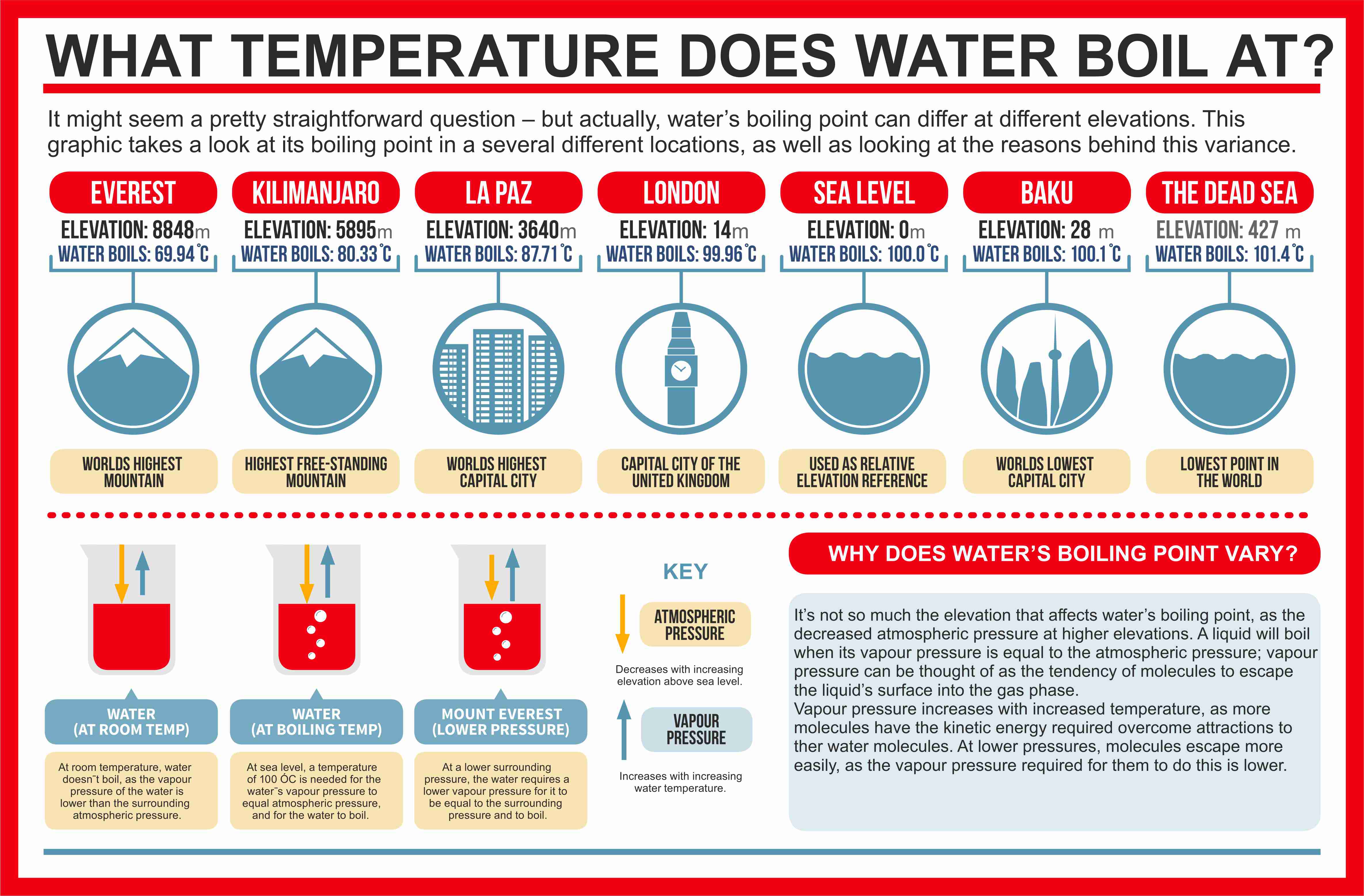
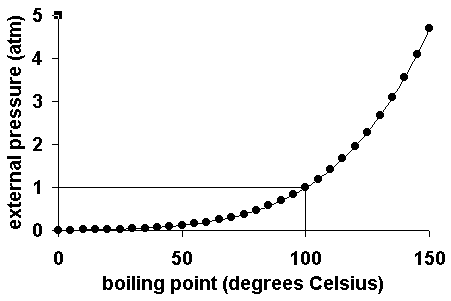
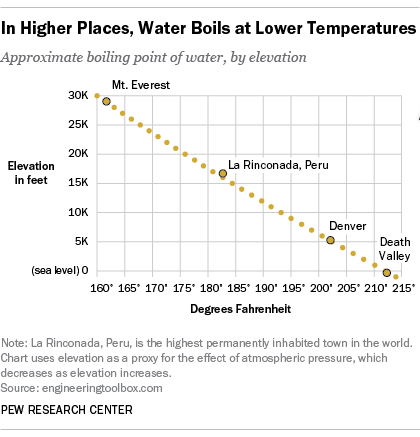
Web at a higher elevation the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e. Web at a higher elevation the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e. Web as you increase your altitude above sea level, the boiling point of water decreases by about 1°f for every 500 feet increase.
You would have to add 58 grams of salt just to raise the boiling. Web how do you lower the boiling point of a liquid? Place a wet cloth on the base and.
The boiling point of water varies with air pressure. At a higher elevation the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e. The more salt (or any solute) added to water, the more.



:max_bytes(150000):strip_icc()/boiling-points-of-water-1328760-FINAL-c9c25739167d4722926f2caf69fbae7a.gif)
/water-in-steel-pan-with-herbs-and-salt-being-added-making-brine-145063802-57a770ea3df78cf459166075.jpg)



/BoilingWater-58dd1c2a5f9b5846837d2a23.jpg)